Abstract
Background: The time to treatment initiation is determined by tumor burden in patients with follicular lymphoma (FL). Tumor burden defined by Groupe d'Etude des Lymphomas Folliculaires (GELF) criteria is one of the widely used to define patients in whom immediate therapy is necessary. GELF criteria are, however, developed in the pre-rituximab era. The definition of tumor burden is not uniform in each clinical trial, and prognosis of patients according to tumor burden has not been well studied. Exploratory analyses were conducted to study clinical relevance of tumor burden defined by GELF criteria in the rituximab era.
Methods: Patients are identified from the list of patients with newly diagnosed FL at our institute from 1999 to 2011. Patients were included if they were diagnosed as clinical stage II to IV, and received first-line R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisolone) based chemotherapy. Three different types of GELF criteria [GELF criteria reported by Brice et al . (J Clin Oncol 1997), GELF1; reported by Ardeshna et al . (Lancet Oncol 2014), GELF2; and reported by Salles et al . (Lancet 2011), GELF3] were evaluated to determine tumor burden predicting clinical outcomes. In the analyses, multiplicity adjustments were not required based on an exploratory research design.
In the group of GELF1, high-tumor-burden was defined if patients had at least the following parameters: tumor mass with a diameter ≥ 7 cm; involvement of three or more nodal sites with a diameter > 3 cm; presence of systemic symptoms; presence of substantial splenic enlargement; presence of serous effusion; presence of local organ compression; or leukemia/cytopenia. In the group of GELF2, high-tumor-burden was defined if at least the following parameters: tumor mass with a diameter ≥ 7 cm; involvement of three or more nodal sites with a diameter > 3 cm; presence of substantial splenic enlargement; presence of serous effusion; or elevated serum lactate dehydrogenase (LDH) levels. In the group of GELF3, high-tumor-burden was defined if at least the following parameters: presence of systemic symptoms; presence of local organ compression; or elevated serum β2-microbloblin in addition to parameters including GELF2.
Results: Among 199 patients, data were available for 92 patients. The median age was 57 years (range 39-79 years) and 42 (46%) patients were male. Twenty-seven patients (29%) had poor risk FLIPI scores and 28 (30 %) had poor-risk FLIPI2 scores. According to GELF criteria, 31%, 35%, and 53% patients were diagnosed as high-tumor-burden defined by GELF1, GELF2, and GELF3, respectively.
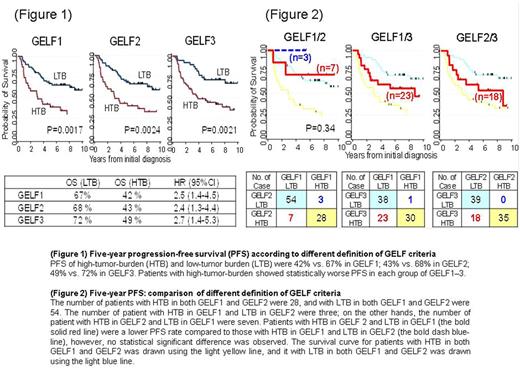
With a median follow-up of 9.3 years, the 5-year overall (OS) and progression-free survival (PFS) rates of all 92 patients were 90% (95%CI: 82 to 95) and 60% (95%CI: 48 to 68). According to three types of criteria, 5-years OS of high-tumor-burden and low-tumor-burden were 77% vs. 97% in GELF1 [hazard ratio (HR) 4.1 (95 % CI 1.3-13.9), P= 0.011]; 77% vs. 98% in GELF2 [HR 4.0 (95 % CI 1.2-13.), P= 0.013]; 83% vs.100% in GELF3 [HR 11.1 (95 % CI 1.4-86.4), P= 0.004]. Five-year PFS of high-tumor-burden and low-tumor burden were 42% vs. 67% in GELF1 [HR 2.5 (95 % CI 1.4-4.5), P= 0.0017]; 43% vs. 68% in GELF2 [HR 2.4 (95 % CI 1.3-4.4), P= 0.0024]; 49% vs. 72% in GELF3 [HR 2.7 (95 % CI 1.4-5.3), P= 0.0021]. Patients with high-tumor-burden showed worse OS and PFS in each of these criteria (Figure 1).
We next compared prognostic value of tumor burden defined by each of these criteria. The number of patients with high-tumor-burden in both GELF1 and GELF2 were 28, and the number of patients with low-tumor-burden in both GELF1 and GELF2 were 54. The number of patient with high-tumor-burden in GELF1 and low-tumor-burden in GELF2 were three; on the other hands, the number of patient with high-tumor-burden in GELF2 and low-tumor-burden in GELF1 were seven. Patients with high-tumor-burden in GELF2 and low-tumor-burden in GELF1 had lower OS and PFS rates compared to those with high-tumor-burden in GELF1 and low-tumor-burden in GELF2, however, no statistical significant difference was observed (Figure 2).
Conclusions: High-tumor-burden defined by three types of GELF criteria (GELF1-3) was associated with worse OS and PFS in patients with FL who received first-line R-CHOP based chemotherapy. Evaluation of tumor burden would be important clinical relevance toward selecting patients who required immediate therapy in the rituximab era.
Kinoshita: Eli Lilly and Kyowa Hakko Kirin: Honoraria. Yamamoto: AbbVie, Celgene, Ono Pharmaceutical, ARIAD Pharmaceuticals, Novartis, Takeda, Eisai, Solasia, MSD, Chugai, Gilead Sciences: Research Funding; ARIAD Pharmaceuticals/CMIC, Celgene, Novartis, Pfizer, Otsuka Pharmaceutical, Takeda, Kyowa Hakko Kirin, Bristol-Myers Squibb, Sumitomo Dainippon Pharma, Ono Pharmaceutical, Mundipharma, Chugai: Honoraria; Ono Pharmaceutical, Meiji Seika Pharma, Novartis, Otsuka Pharmaceutical, Mundipharma, Boehringer Ingelheim: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.
This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal